Trial And Error, Or The Problem Of Clinical Trial Data Exclusivity
Why do we presume that granting patents is the only way to incentivize innovation?
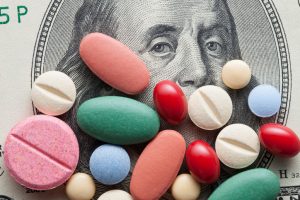 It’s no secret that drug pricing is a huge problem, with pharmaceutical companies doing pretty much everything in their power to maximize profits at the expense of patients. One pharmaceutical company has even gone as far as attempting a sham transfer to a Native American tribe in an attempt to exploit the tribe’s sovereign immunity and protect it from a lawsuit to invalidate its patents.
It’s no secret that drug pricing is a huge problem, with pharmaceutical companies doing pretty much everything in their power to maximize profits at the expense of patients. One pharmaceutical company has even gone as far as attempting a sham transfer to a Native American tribe in an attempt to exploit the tribe’s sovereign immunity and protect it from a lawsuit to invalidate its patents.
The problem with monopoly power is that the rightholder has complete control over the product. The pharmaceutical company can exclude others from producing the drug and, because of the absence of any competition, they can charge whatever prices they want. Sometimes, the rightholder will even raise prices on a life-saving medicine overnight, with no rhyme or reason other than the fact that they can. High profile examples include “Pharma bro” Martin Shkreli raising the price of the medicine Daraprim from $13.5 per pill to $750 per pill overnight, representing a 5,000% increase; Valeant Pharmaceuticals raised the price on the heart medication Isuprel from $180 to $1,472; and under the leadership of the Health and Human Services Secretary Alex Azar, Eli Lilly raised the price of Humalog by 345% during his eight-years at the company. I’ve mentioned before that, at least for medicines where the research was funded by federal grants, the United States government can exercise “march-in” rights to ensure that these inventions are being made “available to the public on reasonable terms.”
But in the case of some pharmaceuticals, even if a bad patent is challenged and invalidated or the United States government actually decided to exercise its “march-in” rights to address pharmaceutical pricing (something that it has never done before), there may still be another barrier: data exclusivity.

Is The Future Of Law Distributed? Lessons From The Tech Adoption Curve
In order to bring a pharmaceutical medicine to market, its efficacy and safety must be proven. Companies invest heavily in clinical trials for new treatments, including for vaccines, drugs and medical devices, to determine whether the product is safe, what the side-effects are, whether the interventions result in positive treatment outcomes and what the risk/benefit ratio is. Clinical trials are a necessary part of the creation and marketing of new drugs—without approval from the Food and Drug Administration, companies can’t sell their medicines—but the way we incentivize them contributes to our current system of exorbitant pricing.
In order to promote investment into clinical trials, which can cost millions of dollars, the United States provides for what is known as “data exclusivity.” Under this system, the company that has invested in the trials can rely on the clinical trial data for a period of years, which differs based on the type of drug, during which period no one else can rely on it. Thus, even if there is no existing patent for a particular drug, if the period of data exclusivity is still valid, a generic company cannot produce and market it because it can’t prove the product is safe.
And why can’t the generic company simply create a new clinical trial and produce its own data to submit to the FDA for approval? Aside from the amount of time and costs associated with clinical trials, there are serious ethical issues because clinical trials involve testing on human subjects. If a drug is already known to be safe and effective, there is no reason to run through a clinical trial again in which ailing patients are given placebos or a less effective medication. The World Medical Association has even set forth the Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects: “Physicians may not participate in a research study involving human subjects unless they are confident that the risks involved have been adequately assessed and can be satisfactorily managed. Physicians must immediately stop a study when the risks are found to outweigh the potential benefits or when there is conclusive proof of positive and beneficial results.” So even if a generic company was willing to invest in clinical trials, medical ethics would prevent such duplication of testing.
We’ve become entrenched in this existing system, with many people thinking that the only way to incentivize investments is through the grant of monopoly. But alternative models do exist. In the case of clinical trials, for example, a section of a trade agreement negotiated by Canada and the E.U. set forth provisions to “avoid duplicative testing on vertebrate animals” and instead “make every effort to ensure that they share tests and studies involving vertebrate animals. The costs of sharing the test and study reports shall be determined in a fair, transparent and non-discriminatory way.” Similarly, several trade agreements between the European Free Trade Association (EFTA)—comprising of Iceland, Liechtenstein, Norway and Switzerland—and countries in Asia and Latin America contain provisions that mandate protection of data but not exclusivity over the data: “Any party may instead allow in their national legislation applicants to rely on such data if the first applicant is adequately compensated.” Under this type of regime, a generic company that wishes to rely on clinical trial data would have to compensate the originator of the data.
Sponsored
How Generative AI Will Improve Legal Service Delivery


Early Adopters Of Legal AI Gaining Competitive Edge In Marketplace

Legal AI: 3 Steps Law Firms Should Take Now

Legal AI: 3 Steps Law Firms Should Take Now
Senator Bernie Sanders has introduced this idea in standalone bills and as an amendment to other bills since at least 2010, but this idea has had no traction in Congress. Instead, we seem to be stuck in this terrible system in which we rely solely on monopoly incentives, sometimes at the cost of human lives.
Krista L. Cox is a policy attorney who has spent her career working for non-profit organizations and associations. She has expertise in copyright, patent, and intellectual property enforcement law, as well as international trade. She currently works for a non-profit member association advocating for balanced copyright. You can reach her at kristay@gmail.com.
Sponsored

Navigating Financial Success by Avoiding Common Pitfalls and Maximizing Firm Performance








